January 03, 2025
4 min read
A 48-year-old man presented to the Lahey Eye Clinic as a new patient for care after recent head trauma while he was in another state.
He was experiencing constant headaches, he felt his eyes looked “swollen and infected,” and vision was poor in the right eye.
Source: Ke Zeng, MD, and Laurel Vuong, MD
Three weeks prior, he was admitted to an outside hospital after an electric scooter accident. He was not wearing a helmet. He was found to have a subdural hemorrhage, right retrobulbar hemorrhage, and fractures involving the zygomatic arches bilaterally, right orbital floor and left lateral orbital wall. Ophthalmology evaluated him and ruled out entrapment and orbital compartment syndrome.
During his hospital course, repeat CT and MRI of the head were obtained because of persistent headaches, but no new findings were seen. There were normal flow voids in both superior ophthalmic veins and cavernous sinuses. His course was complicated by right facial droop and dysarthria. He developed pulmonary embolism, for which he was discharged on apixaban.
His medical history included hypertension and depression. His ocular history included myopic LASIK in both eyes.
Examination at current presentation
Visual acuity could not be obtained in the right eye and was 20/150 in the left eye. IOPs were 15 mm Hg in the right eye and 13 mm Hg in the left eye. There was a right relative afferent pupillary defect. Extraocular motility was severely limited bilaterally.
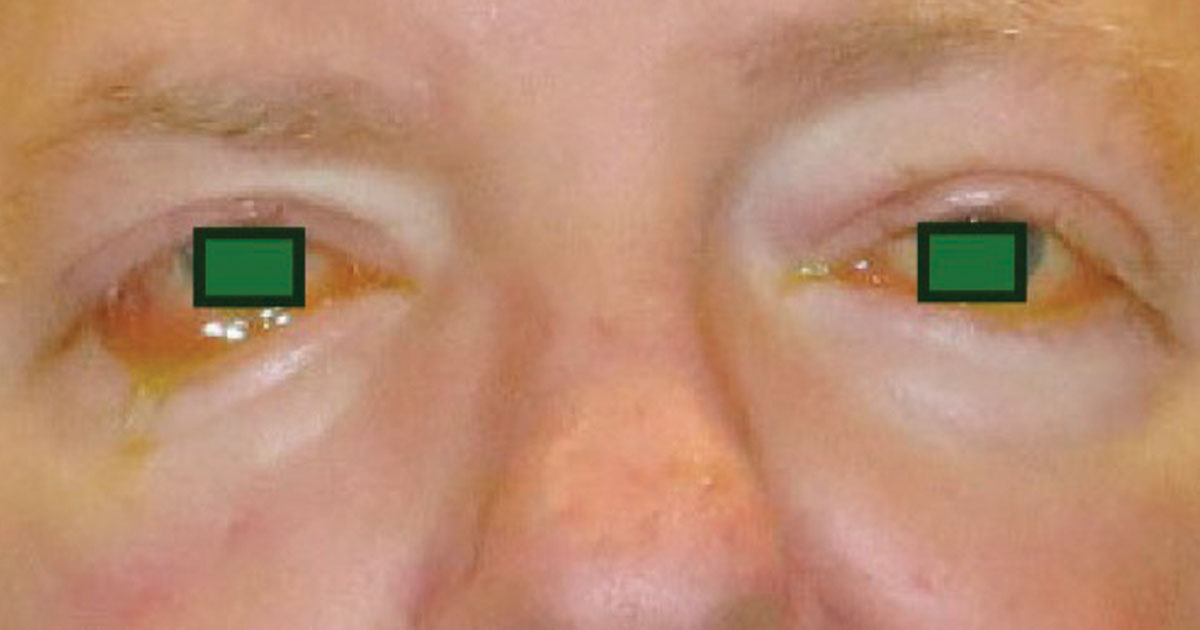
Exophthalmometry measurements were 34 mm in the right eye and 33 mm in the left eye. A right facial droop was observed, along with right-sided ptosis greater than left-sided ptosis. He had significant inferior chemosis bilaterally (Figure 1). Dilated fundus exam showed right optic disc pallor with no other findings.
What is your diagnosis?
See answer below.
Proptosis, ophthalmoplegia and chemosis
In the setting of bilateral proptosis, ophthalmoplegia and severe chemosis along with decreased vision after trauma, there would be concern for a traumatic carotid-cavernous fistula. The differential diagnosis would also include cavernous sinus syndrome due to cavernous sinus thrombus or superior ophthalmic vein thrombus. However, this would be unlikely given that the patient was on an anticoagulant. Other considerations include orbital cellulitis given his recent sinus fractures, but he did not have signs of an infection or fever. Traumatic cranial nerve palsy and late-presenting entrapment from orbital fractures are lower on the differential as they would not cause proptosis or severe chemosis.
Workup and management
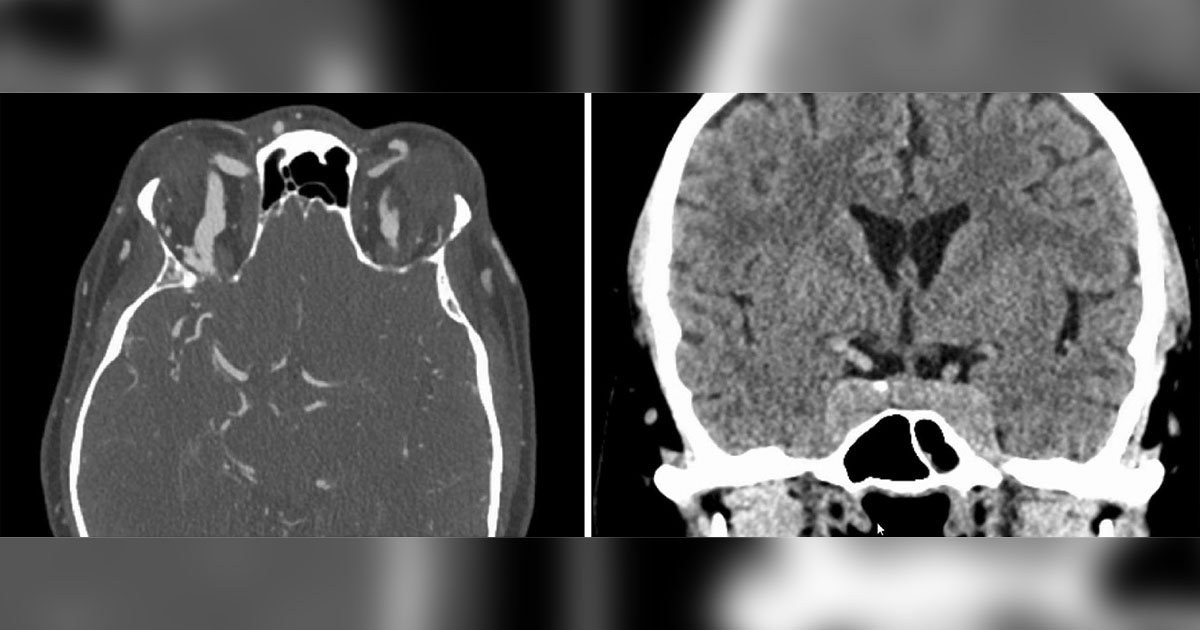
The patient was immediately sent to the emergency department for an urgent workup. CT and CTA of the head showed enlargement of both superior ophthalmic veins and bulging of the cavernous sinuses (Figure 2). The facial fractures were unchanged. Subsequent diagnostic cerebral angiography revealed a right direct carotid-cavernous sinus fistula.
Discussion
A carotid-cavernous fistula (CCF) is due to an abnormal connection between the carotid artery and/or its branches and the cavernous sinus. Arterial blood with relatively higher pressures entering the lower pressure venous system leads to impaired orbital and ocular drainage. Seventy percent of CCF result from trauma, although spontaneous fistula can also form. Direct CCF, which forms between the internal carotid artery and the cavernous sinus, has a high flow and often presents days to weeks after trauma. Indirect CCF, also referred to as a dural shunt, which forms between branches of the carotid artery and the cavernous sinus, typically occurs in patients with hypertension and older women. This has a low flow and often presents more insidiously.


The symptoms of CCF include diplopia, blurred vision, orbital pain and headaches. Impaired ocular venous drainage can cause chemosis, dilated “corkscrew” episcleral vessels, elevated IOP possibly causing glaucoma, retinal vein occlusion, venous stasis retinopathy and/or ischemic optic neuropathy. Impaired orbital venous drainage can cause ocular bruit, proptosis and ophthalmoplegia, which is present in 63% of direct CCF. Unilateral direct CCF can present with bilateral symptoms. If the pressure rise in the eye and orbit is sudden, angle-closure glaucoma may occur.
In-office testing methods for CCF include ultrasound that can show superior ophthalmic vein dilation or flow reversal with color Doppler imaging. Increased mires movement on applanation compared with the contralateral eye may be helpful. Other methods include ocular bruit that worsens with the Valsalva maneuver. Lastly, an ocular pulse amplitude (systolic IOP minus diastolic IOP) on pneumotonometry that is greater than 1.6 mm Hg between the two eyes is 93% sensitive.
CCFs are ultimately diagnosed via CTA or MRA. A direct connection between the carotid artery and the cavernous sinus is confirmatory. However, dilation of the cavernous sinus, ophthalmic veins and extraocular muscles is a nonspecific finding that should raise concern.
Depending on the patient’s symptoms and visual function, CCF can be observed, managed conservatively, or repaired by endovascular embolization or stereotactic surgery. Up to 70% of indirect CCFs close spontaneously. Conservative measures such as carbonic anhydrase inhibitors and beta-blockers can decrease IOP. Manual compression of the internal carotid artery has also been suggested to slow blood flow, allowing fistula closure. Transarterial embolization with balloons, coils or stents is favored for direct CCF repair. Indirect CCF is more commonly repaired through a transvenous approach.
Patient follow-up
The patient underwent urgent embolization and flow reversal of the fistula. At 1-month follow-up, the chemosis and proptosis had resolved (Figure 3). Vision in the right eye was counting fingers, but vision in the left eye improved to 20/20. He had an improving corneal dellen in the right eye, and there was right paralytic lagophthalmos.
- References:
- Debnam JM, et al. Cavernous sinus. In: Debnam JM, ed. Imaging Atlas of Ophthalmic Tumors and Diseases. Springer; 2023:279-307.
- Gupta AK, et al. Neuroradiology. 2006;doi:10.1007/s00234-006-0132-x.
- Henderson AD, et al. Eye (Lond). 2018;doi:10.1038/eye.2017.240.
- Loggini A, et al. Front Neurol. 2022;doi:10.3389/fneur.2021.715955.
- For more information:
- Edited by William W. Binotti, MD, and Julia Ernst, MD, PhD, of New England Eye Center, Tufts University School of Medicine. They can be reached at william.binotti@tuftsmedicine.org and julia.ernst@tuftsmedicine.org.

Leave a Reply