December 20, 2024
9 min read
A 24-year-old previously healthy Greek man with recent COVID-19 infection presented to the Tufts emergency department with a 1-week history of high fevers, generalized weakness, sore throat and blurry vision.
On systemic review of systems, the patient was positive for fever, malaise, fatigue, cough, hemoptysis, palpitations, one episode of vomiting, headache and dizziness. Other reviews of systems were negative. The patient had no other medical history, surgical history, medications, ocular history or allergies.
Source: Virali Shah, MD, and Shilpa Desai, MD
On ocular review of systems, the patient was positive for blurry vision in both eyes (worse in the left eye than the right eye), floaters in both eyes and flashes in the left eye. Family history was positive for non-Hodgkin’s lymphoma (paternal) and prostate cancer (paternal). On social history, the patient had recently moved from Oregon to Boston for a software engineering job. He was an active smoker (smoke history of 10 cigarettes per day) and a social drinker. He used marijuana about once per week; he denied all other drug use.
Examination
On examination, the patient presented to the ER with the following vital signs: temperature of 99.8°F, heart rate of 115 beats per minute, blood pressure of 117/73 mm Hg, respiratory rate of 20 breaths per minute and oxygen saturation of 98% on room air. He was awake, alert and oriented to person, place and time. He had no pain.


Initial ocular exam revealed near vision of 20/100 in the right eye with no improvement on pinhole and counting fingers at 1 foot in the left eye with no improvement on pinhole. IOP was 13 mm Hg in both eyes. Pupils were equally round and reactive. Visual fields and extraocular movements were full in both eyes. Color vision was intact in the right eye but absent in the left eye. Slit lamp biomicroscopy exam was within normal limits in both eyes.
Dilated fundus exam revealed some notable findings. In the right eye, the disc margins were well defined with a small cup-to-disc ratio. There was no evidence of edema or signs of infiltration. The macula showed preretinal and intraretinal hemorrhages (Figure 1). The fovea appeared intact with some perifoveal heme. Vessels were tortuous, and the periphery also showed scattered intraretinal hemorrhages inferior, inferonasal, superior and superotemporal. There were also some white-centered hemorrhages, also known as Roth-like spots. Similar to the right eye, the disc margins of the left eye were well defined with a small cup-to-disc ratio. There was extensive preretinal heme covering the fovea up to the disc (Figure 1). Vessels were tortuous without apparent plaque or embolus. The periphery showed scattered intraretinal heme with few white-centered hemorrhages.
Initial workup
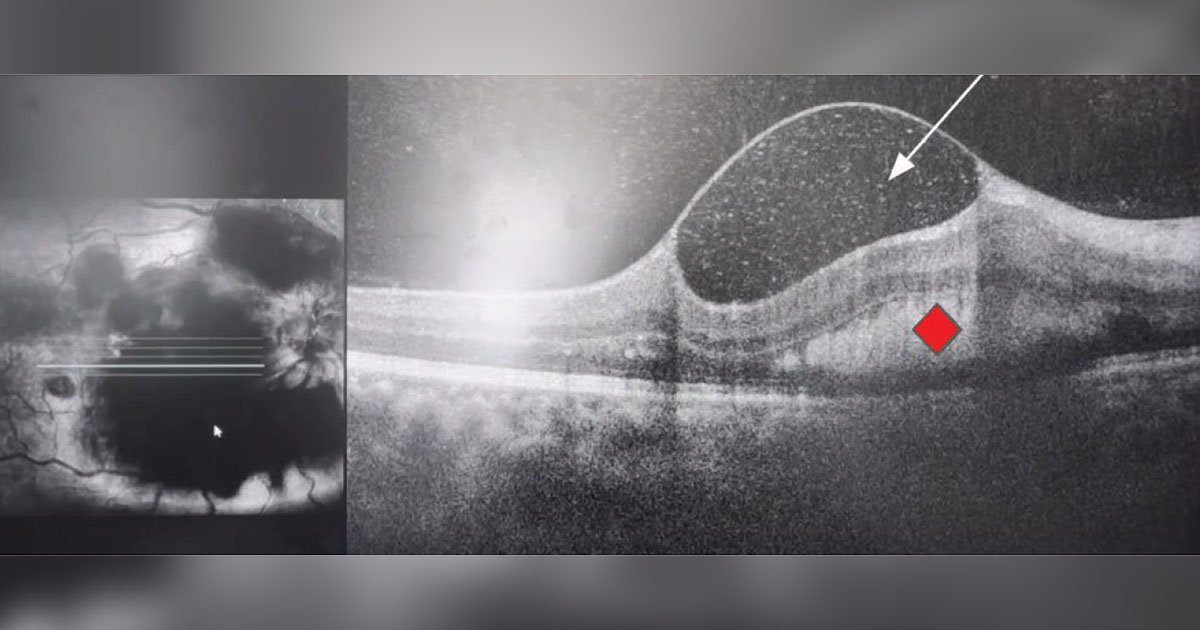
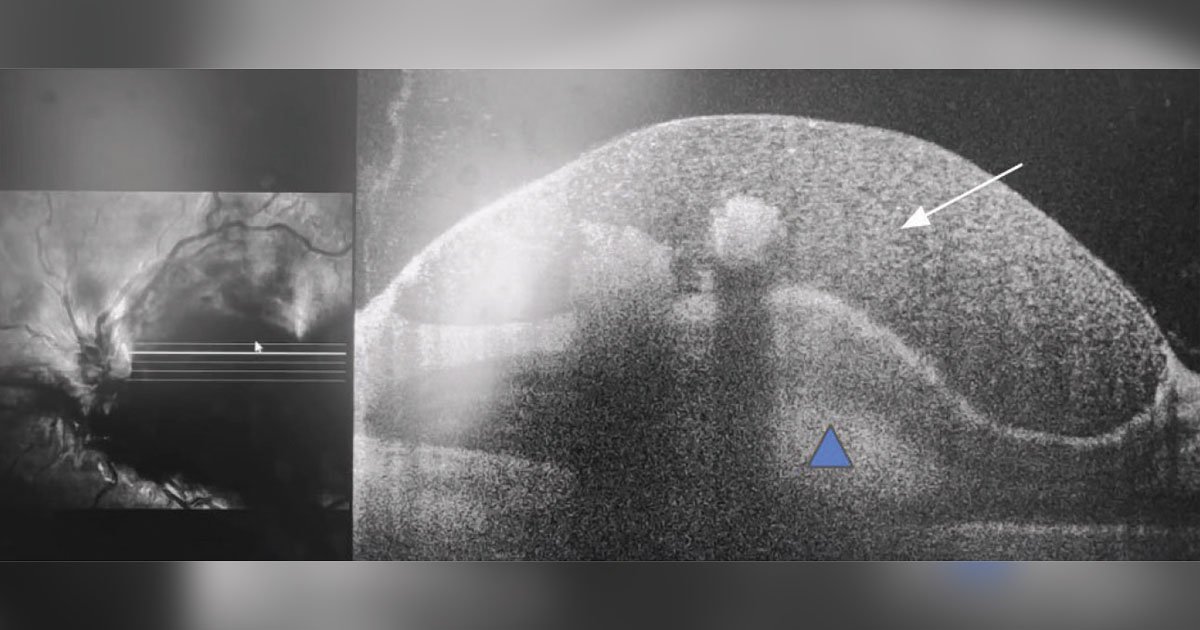
Arriving at an accurate diagnosis can be aided with the use of various imaging modalities. To further evaluate the hemorrhages and foveal findings from the dilated fundus exam, color fundus photography and OCT of the macula were obtained. Fundus photography of both eyes highlighted the aforementioned findings on exam. OCT of the right eye showed some vitreous opacity, which may have indicated some vitreous heme (Figure 2). There was sub-internal limiting membrane heme and subretinal hyperreflective material, which also represented the heme seen on exam. OCT of the left eye showed some preretinal heme and intraretinal hyperreflective material (Figure 3).
What is your diagnosis?
See answer below.
Multilayered retinal hemorrhages
Returning to the patient’s initial presentation of blurry vision with multilayered retinal hemorrhages on ocular exam, it is important to keep a broad differential in these cases. In regard to the patient’s multilayered retinal hemorrhages, the differential diagnosis includes diabetic retinopathy, hypertensive retinopathy, central retinal vein occlusion, ruptured arterial macroaneurysm, non-accidental trauma, anemia and thrombocytopenia.
The patient did not have a history of diabetes or hypertension, suggesting vascular causes of the multilayered hemorrhages, including ruptured arterial macroaneurysm, were less likely. He denied a sudden increase in intrathoracic or intra-abdominal pressure in the preceding weeks, as is seen in Valsalva retinopathy. In addition, intraretinal and subretinal heme is noted less commonly in Valsalva retinopathy. Terson’s retinopathy is associated with subarachnoid hemorrhage, intracranial hemorrhage or traumatic brain injury. This patient’s CT and MRI ruled out any aneurysm or intracranial or extracranial bleed. Non-accidental trauma also presents as multilayered hemorrhages, but this patient did not have any history of trauma and did not have any fractures or injuries on physical exam.
Further workup
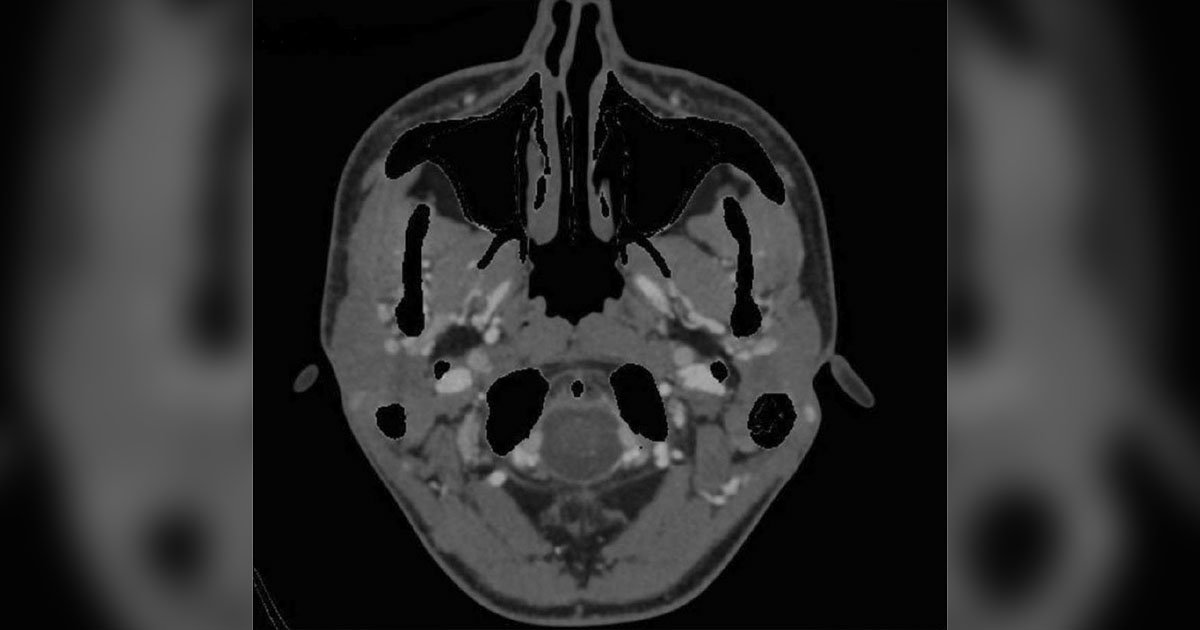
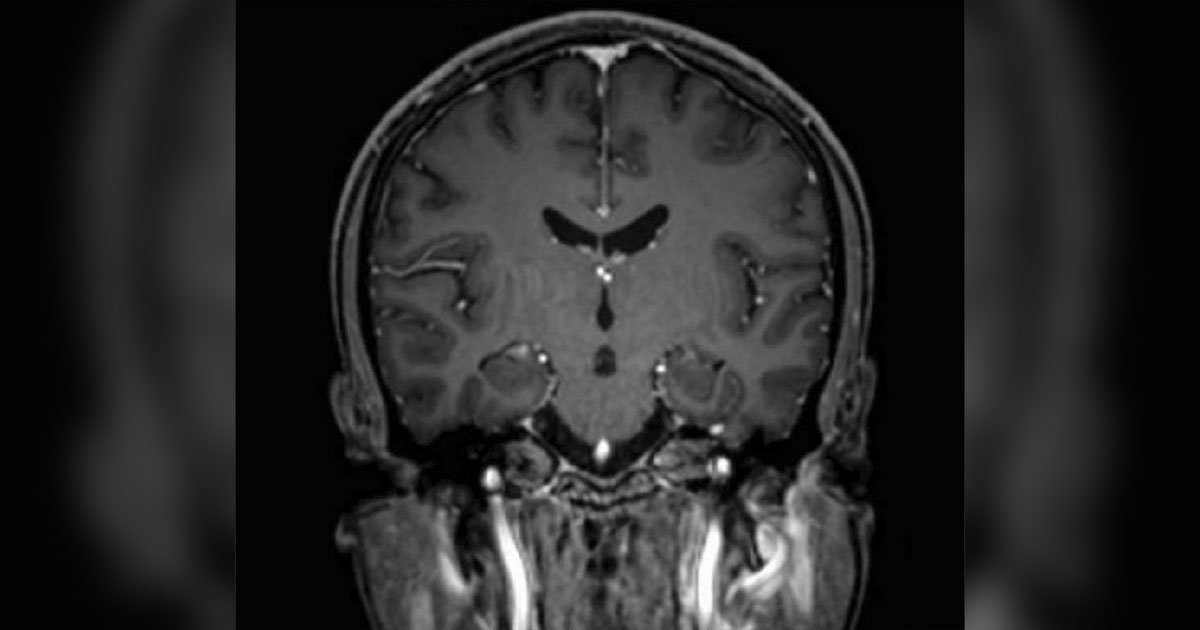
Due to the concern for infiltration and multilevel hemorrhages in the retina, the decision to undergo systemic workup with further imaging and bloodwork was taken. A CTA of the head and neck was ordered and ruled out aneurysm, intracranial hemorrhage and compressive lesion (Figure 4). The only notable finding was an enlarged 1.8 cm lymph node in the left jugular chain. MRI of the head further revealed few scattered hyperintense foci bilaterally in the frontal subcortical and periventricular white matter (Figure 5); otherwise, it was consistent with the CTA results. Initial labs included the following results: Complete blood count showed leukocytosis with a white blood cell (WBC) count of 65.8, anemia with a hemoglobin of 4.3 and thrombocytopenia with a platelet count of 18. Comprehensive metabolic panel was mostly within normal limits except for mild hypokalemia at 3.3. Infectious workup was negative, which included blood cultures, MRSA culture, influenza virus test, SARS-CoV-2 test and QuantiFERON-TB Gold test. EKG revealed sinus tachycardia. Urine analysis ruled out a urinary tract infection.
For the prominent leukocytosis in the setting of systemic symptoms, the patient’s primary team ordered a peripheral blood smear to analyze the WBCs. His final smear showed blast cells and a few schistocytes, which was followed up with an urgent bone marrow biopsy and flow cytometry test. The core biopsy showed 90% myeloid blast cells in the cell differential, and the aspirate smear was positive for Auer rods. Flow cytometry tagged these cells positive for CD45.
The patient’s diagnosis was leukemic retinopathy in the setting of newly diagnosed acute myeloid leukemia.
Management
After diagnosis, the patient was admitted to the hematology/oncology service of the hospital and started immediately on induction chemotherapy (7+3 C1D1). He was regularly transfused to resolve the hematologic abnormalities and underwent genetic testing that revealed trisomy 8 and KRAS/FLT3 positivity, which led to modification in his chemotherapy regimen (transition to midostaurin).
For the ophthalmology team, the main priorities for his inpatient ocular management in leukemia was close follow-up with repeated exams and encouraging treatment of his underlying leukemia.
Discussion
Leukemia commonly manifests as fatigue, bleeding and fever. However, it is important to note that there are other times when leukemia’s initial symptom may be blurry vision or even vision loss, such as in this patient. When leukemia affects the eye, the first target is the retina. The pathophysiology of leukemic retinopathy is broken down into two main processes: primary and secondary. Primary ocular involvement in leukemia involves actual neoplastic cell infiltration and invasion of the retina, orbit, choroid and/or optic nerve. The internal limiting membrane serves as an effective barrier against invasion of these neoplastic cells to other parts of the eye. However, if this barrier is interrupted, there can be infiltration into the vitreous. If leukemic cells invade the choroid, there may be reduced blood flow in the choriocapillaris and altered retinal pigment epithelium function, leading to accumulation of subretinal fluid or even a serous retinal detachment. Cells can also directly infiltrate the optic nerve head, appearing as gray-white streaks of infiltrates around the disc. Primary neoplastic infiltration is observed in about 3% of leukemic cases.
Secondary or indirect ocular processes of leukemic retinopathy involve the hematologic abnormalities that develop in leukemia, such as anemia, thrombocytopenia and leukocytosis. The indirect pathway of leukemic retinopathy is much more common in patients, with up to 50% of leukemic cases manifesting some degree of indirect ocular changes.
In the acute stages of leukemia, a combination of vessel wall injury, anemia and thrombocytopenia causes multilayered retinal hemorrhages, which we saw manifested diffusely and bilaterally in our patient. Other retinal manifestations such as cotton wool spots and white-centered hemorrhages are also common.
In the more chronic setting, leukostasis retinopathy can develop. This is where leukocytosis causes the blood to become hyperviscous. Over long periods of time, the hyperviscous blood becomes more stagnant, and vascular stasis can occur. Vascular stasis causes a focal increase in platelet count, which can further cause blood occlusion and ischemia. When this happens in the retina, it can lead to peripheral retinal ischemia, causing devastating and blinding complications such as microaneurysms, proliferative neovascular retinopathy and even central retinal vein occlusion.
Because of the multiple pathways in which leukemia can affect the eye, there is a diverse range of retinal manifestations. In this patient and in most patients with leukemia, the most noteworthy finding is the multilayered retinal hemorrhages. Other findings may also include leukemic infiltrates, cotton wool spots, central retinal vein occlusion and white-centered hemorrhages (as seen in this patient). True white-centered hemorrhages (Roth spots) are typically associated with infective endocarditis and manifest due to retinal endothelial dysfunction. Leukemic white-centered hemorrhages (Roth-like spots) may manifest similarly on exam, but they are due to accumulation of leukemic cells at the center causing the white appearance.
The prognosis for patients with leukemic retinopathy is associated with more aggressive forms of leukemia and worse overall outcomes. Prior research has found that the 5-year survival rate was significantly shorter in those with ocular involvement than those without and that patients who specifically had cotton wool spots had higher odds of mortality. In terms of visual prognosis, it depends on the degree of leukemic retinopathy. Mild retinopathy, such as retinal hemorrhages and dilated tortuous vessels, will resolve slowly with reabsorption and as the underlying leukemia is treated with chemotherapy, radiation or surgery. As hemorrhages slowly reduce in size, visual acuity will gradually improve as well. In moderate or severe forms of leukemic retinopathy, permanent visual damage is more common.
The main goal for these patients is treating the underlying leukemia, usually with chemotherapy. Leukapheresis and bone marrow transplants are also part of adjunctive therapy. Patients with severe forms of leukemic retinopathy may need intraocular treatment, which can include radiation to decrease intraocular infiltrates, pars plana vitrectomy if there is retinal detachment or massive subretinal hemorrhages, or anti-VEGF injections if there is proliferation or edema. In recent years, there has also been explorative research in intravitreal chemotherapy agents such as methotrexate.
Clinical course continued
The patient’s hematologic abnormalities gradually resolved after chemotherapy and as-needed transfusions. On day 14 of inpatient follow-up, he started showing some visual improvement in his right eye with a visual acuity of 20/200 from 20/400. His dilated fundus exam and repeat color fundus photos showed reduced amounts of vitreous and preretinal heme with resolution of most Roth-like hemorrhages (Figure 6).
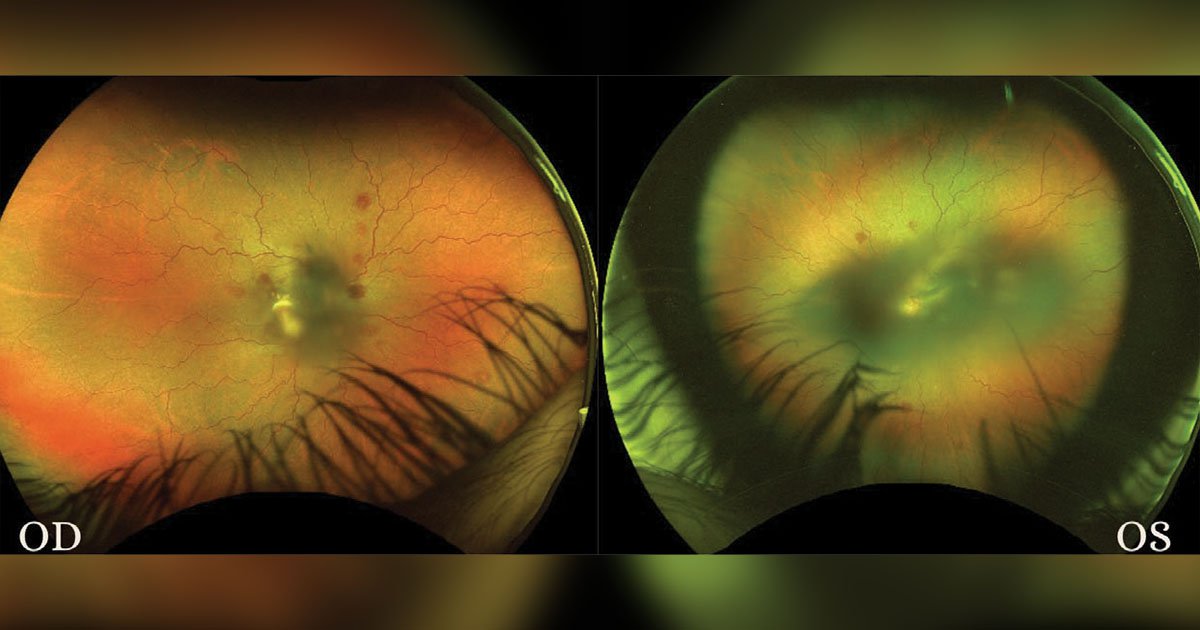
On day 21 of inpatient follow-up, he continued to have a stable visual acuity of 20/200 in the right eye and counting fingers in the left eye. His dilated fundus exam, however, continued to show decreased size and amounts of heme. Nearing the end of his inpatient admission, the patient had completed 3 weeks of inpatient chemotherapy. He received leukapheresis and transfusions as needed. His final post-induction bone marrow biopsy revealed no blasts, so he was discharged with a plan to receive a bone marrow transplant and continue chemotherapy management at Memorial Sloan Kettering Cancer Center.
- References:
- Abu el-Asrar AM, et al. Doc Ophthalmol. 1995;doi:10.1007/BF01204178.
- Allen RA, et al. Arch Ophthalmol. 1961;doi:10.1001/archopht.1961.00960010492010.
- Arlotti SA, et al. J AAPOS. 2007;doi:10.1016/j.jaapos.2006.09.023.
- Balakrishnan U, et al. Multilayered hemorrhage in acute myeloid leukemia (AML). https://eyerounds.org/cases/317-leukemic-retinopathy.htm. Published Jan. 20, 2022. Accessed Nov. 22, 2024.
- Bhende MP, et al. Indian J Ophthalmol. 2013;doi:10.4103/0301-4738.112163.
- Curto ML, et al. Med Pediatr Oncol. 1989;doi:10.1002/mpo.2950170212.
- Czorlich P, et al. Neurosurg Rev. 2015;doi:10.1007/s10143-014-0564-4.
- Fountas KN, et al. J Neurosurg. 2008;doi:10.3171/JNS/2008/109/9/0439.
- Ginat DT, et al. Neurology. 2015;doi:10.1212/WNL.0000000000001723.
- Hui VWK, et al. Clinical and imaging features of leukemic retinopathy. In: Guenova M, et al, eds. Leukemia: From Biology to Clinic. IntechOpen; 2023;doi:10.5772/intechopen.107649.
- Jiang X, et al. Front Med (Lausanne). 2023;doi:10.3389/fmed.2023.1051089.
- Murugesan N, et al. Semin Thromb Hemost. 2015;doi:10.1055/s-0035-1556731.
- O’Malley E, et al. Central retinal artery occlusion (CRAO): 81-year-old white male with sudden, painless vision loss in left eye. http://www.eyerounds.org/cases/case20.htm. Published Feb. 21, 2005. Accessed Nov. 22, 2024.
- Raevis J, et al. Am J Ophthalmol Case Rep. 2020;doi:10.1016/j.ajoc.2020.100993.
- Sharma T, et al. Eye (Lond). 2004;doi:10.1038/sj.eye.6701308.
- Simakurthy S, et al. Valsalva retinopathy. In: StatPearls [Internet]. StatPearls Publishing. Updated Aug. 25, 2023.
- Speilburg AM, et al. J Optom. 2014;doi:10.1016/j.optom.2013.08.002.
- Togioka BM, et al. J Emerg Med. 2009;doi:10.1016/j.jemermed.2008.06.022.
- Vishnevskia-Dai V, et al. Sci Rep. 2020;doi:10.1038/s41598-020-58654-8.
- Wu L, et al. Ophthalmologic manifestations of leukemias treatment & management. https://emedicine.medscape.com/article/1201870-treatment. Published July 22, 2022. Accessed Nov. 22, 2024.
- For more information:
- Edited by William W. Binotti, MD, and Julia Ernst, MD, PhD, of New England Eye Center, Tufts University School of Medicine. They can be reached at william.binotti@tuftsmedicine.org and julia.ernst@tuftsmedicine.org.

Leave a Reply